Gas Chromatography (GC)
Gas chromatography (GC) is a widely used analytical method for separating and analyzing volatile and semi-volatile compounds present in a mixture. This technique is favored due to its excellent ability to resolve compounds quickly and sensitively. GC finds extensive applications in various industries, including environmental, petroleum, chemical, food and beverage, and pharmaceutical.
Similar to other chromatographic methods, GC involves a stationary phase and a mobile phase. In this case, the mobile phase is an inert gas, typically helium or nitrogen, while the stationary phase can be either a solid adsorbent, known as gas-solid chromatography (GSC), or a liquid adsorbed onto an inert support, known as gas-liquid chromatography (GLC, or just GC).
HOW DOES GAS CHROMATOGRAPHY OPERATE?
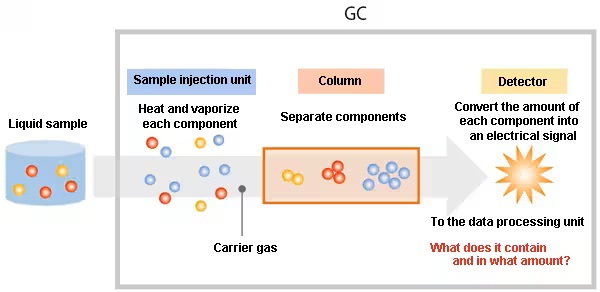
Once the sample is collected and prepared, the target analytes are separated within the column, and a detector measures the quantity of components that exit the column. In GC, the analyte is introduced into the sampling port of the instrument and then vaporized inside an oven. The resulting vaporized sample is carried through the chromatographic column by an inert gas, serving as the mobile phase. Compounds in the sample distribute between the column’s stationary phase and the carrier gas. The strength of interaction between a compound and the stationary phase determines its retention time. As compounds pass by the column’s outlet, a detector (either MS or non-MS) generates a signal. The result of a GC separation is represented in a chromatogram.
To determine the concentration of a test sample, a standard sample with a known concentration is also injected into the GC instrument. The peak retention time and area of the standard are then compared to the test sample’s results to deduce the unknown concentration. In GC, external and internal standards are commonly utilized to ensure reliable quantification of the test sample. When known standards are separately analyzed from the actual sample of interest, and their response is compared to that of the sample in another chromatogram, it is termed an external standard. On the other hand, if the standard is added to the sample and analyzed simultaneously, it is referred to as an internal standard.
SAMPLE COLLECTION AND PREPARATION FOR GC ANALYSIS
To conduct GC analysis, a wide range of samples can be used as long as the compounds are sufficiently volatile to be vaporized and thermally stable enough not to degrade at high temperatures. These samples can originate from solid, liquid, or gaseous materials. During sample preparation for GC, it is crucial to reduce sample complexity, as the quality of the sample significantly influences the accuracy and precision of the final chromatographic results. To achieve this, there are various matrix-specific sample preparation techniques available, which aid in isolating and concentrating analytes and sample matrices before subjecting them to GC analysis.
CHOOSING A GC COLUMN
The GC column serves as the core of a GC system, facilitating chromatographic separation. Opting for the right GC column involves considering four crucial factors: stationary phase, column internal diameter (I.D.), film thickness, and column length. These factors play a vital role in determining column efficiency, resolution, and sample capacity. The selection of the stationary phase should be based on the specific application to be carried out.

